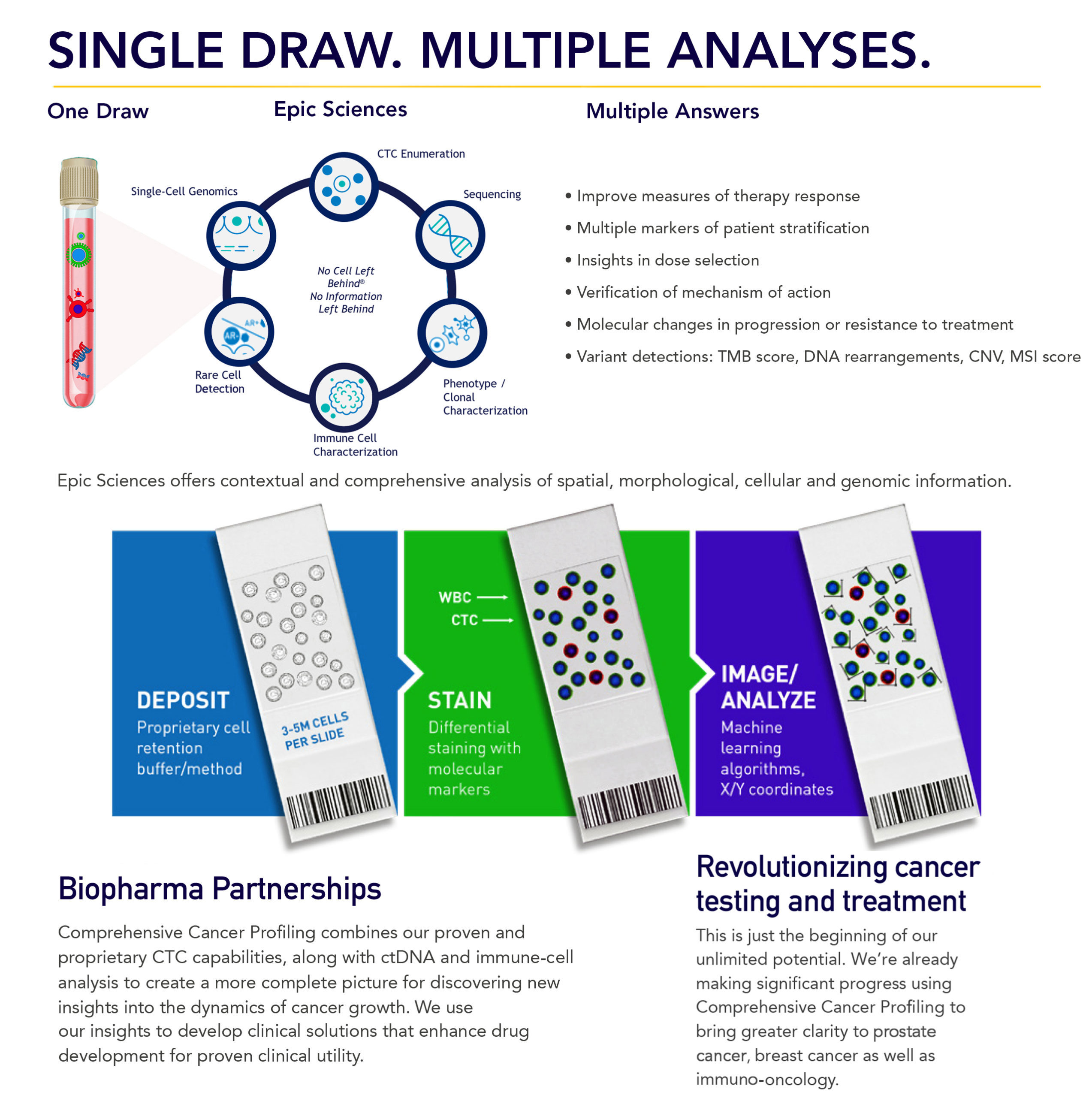
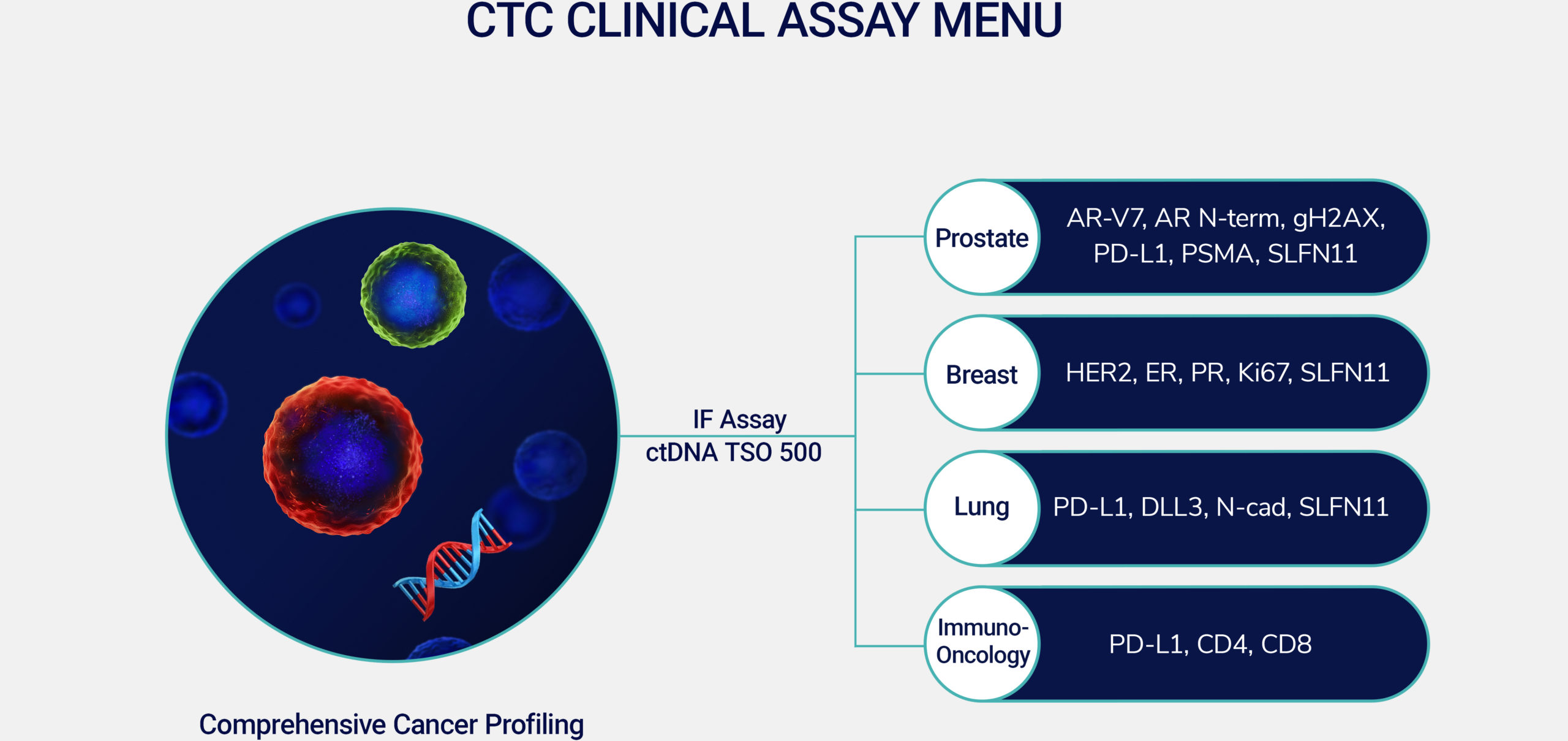
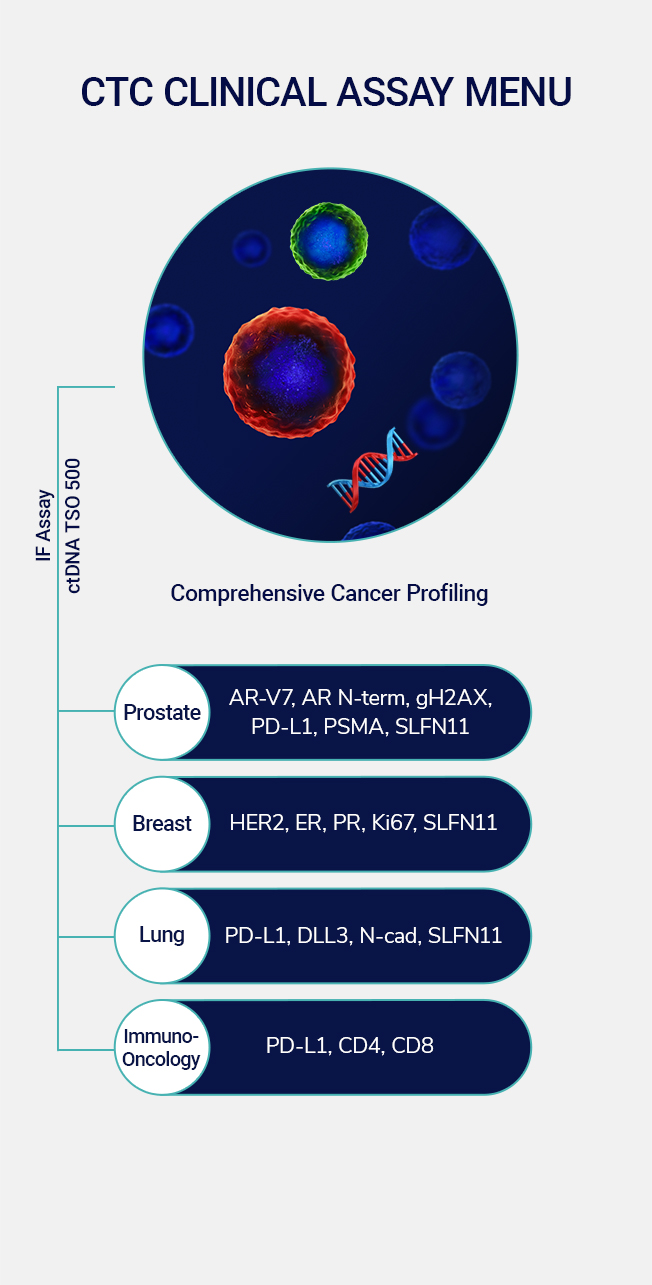
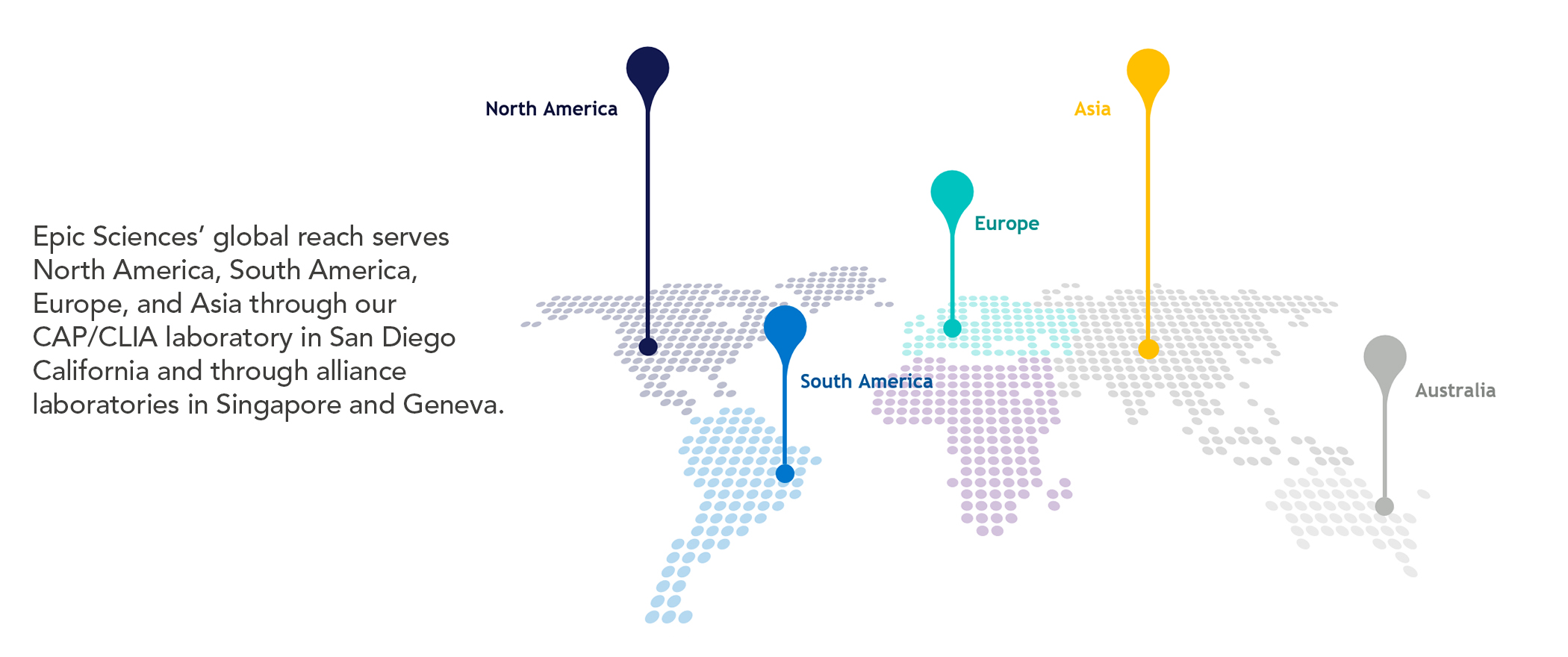
Our clinical solutions, proven by partnerships, help enhance drug development and expedite commercialization with valuable insights into the dynamics of cancer growth and evolution. Comprehensive Cancer Profiling combines our proven and proprietary CTC capabilities, along with ctDNA and immune-cell analysis to create a more complete picture for discovering new insights into the dynamics of cancer growth. We use our insights to develop clinical solutions that enhance drug development for proven clinical utility.